Uncut sheet for lgG/igM Antibody to Dengue
1. INTEDED USE
This kit is used for in vitro qualitative detection of IgG/IgM antibody to dengue in human whole blood, serum or plasma sample, which's applicable to auxiliary diagnosis of dengue virus infection. This kit only provides detection results of IgG/IgM antibody to dengue, and results obtained shall be used in combination with other clinical information for analysis. This kit is for healthcare professionals
2. PRODUCT SPECIFICATION
| Model No. | Dengue lgG/lgM AntibodyUncut sheet |
| Methodology | Colloidal Gold |
| Sample Type | whole blood/Serum/Plasma |
| Time to Result | 15~20mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485, CE Certificate, UCKA MHRA Certificate |

|
Feature • High sensitive • result reading in 15-20 minutes • Easy operation • High Accuracy |
PACKING * 20 bags /CTN * Aluminum foil bag labeling * shrink wrap
|
3.TEST METHOD
| 1 | Remove detection reagent and sample to be detected from storage condition and equilibrate them to room temperature. |
| 2 | Open aluminum foil pouch of reagent, take out test device, and place it horizontally on laboratory bench; |
| 3 | Use disposable pipette provided to pipette sample. lf serum or plasma sample is usedD2one can directly and vertically add 8 drops (approx 80 μL) to "D1" well without addingsample diluent and start counting time. lf whole blood sample is used, vertically add 8drops (approx 80 uL) to "D1" well, then term sample diluent upside down, discard first 2drops of sample diluent, vertically and slowly add 1 drop of bubble-free sample diluent dropwise to "D1" wel, and start counting time. |
| 4 |
Result shall be interpreted within 15~ 20 minutes, and detection result is invalid after 20 minutes.
|
4.RESULT EVALUATION AND EXPLANATION
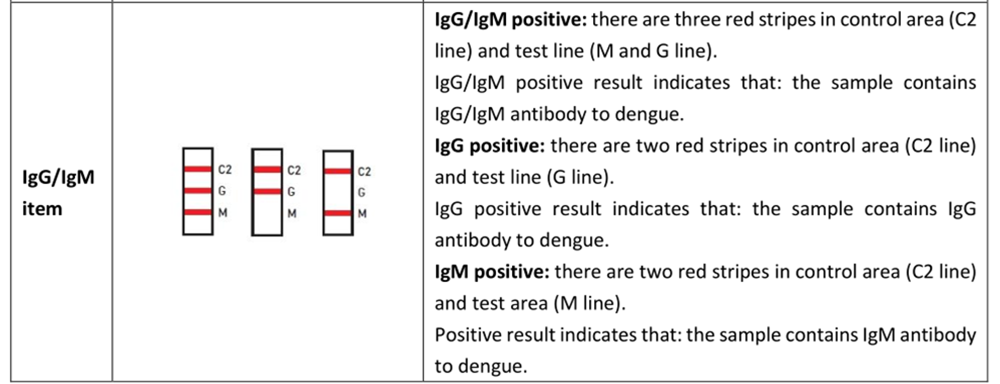
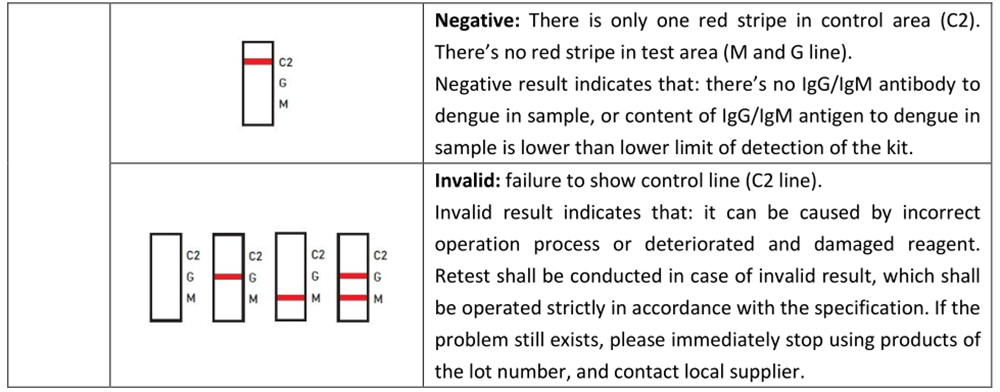
5. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration