Diagnostic Kit for Follicle Stimulating Hormone
1. INTEDED USE
This kit is intended for in vitro quantitative detection on the content of follicle-stimulating hormone (FSH) in the human serum/plasma/blood samples and is intended for assisting in judging the occurrence of female climacteric period. This kit only provides the test result of follicle-stimulating hormone (FSH), and the obtained result shall be analyzed in combination with other clinical information.
2. PRODUCT SPECIFICATION
| Model No. | FSH |
| Methodology | Colloidal Gold |
| Sample Type | URINE |
| Time to Result | 10-15 minutes. |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE |
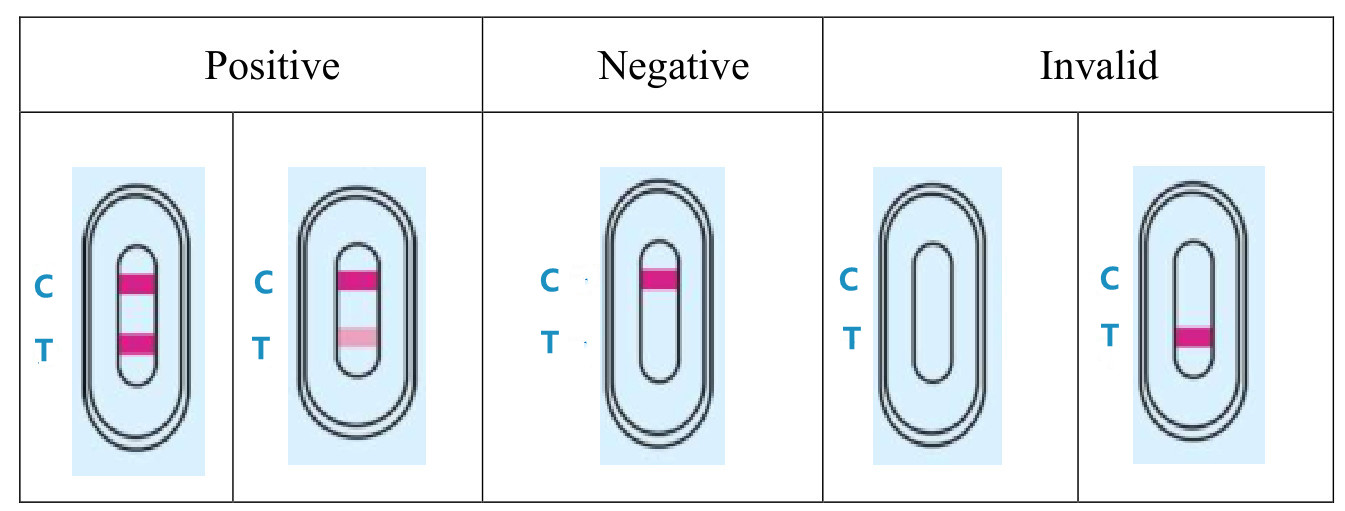
3.RESULT EVALUATION AND EXPLANATION
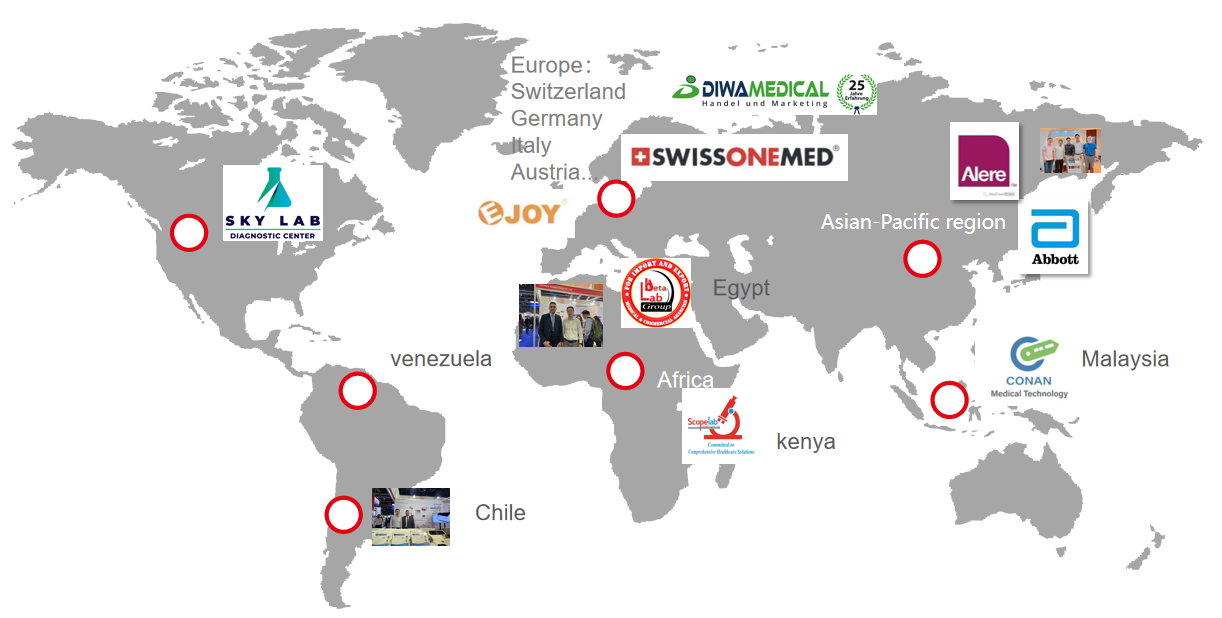
Baysen medical take part in all kinds of exhibition to establish more cooperation with clients all over the world.
We aim to provide a more healthier life for human being in the world.
5. GLOBAL PARTNER
Write your message here and send it to us






