Diagnostic Kit for tetrahydrocannabinol Colloidal Gold
1. INTEDED USE
This kit is applicable to qualitative detection of tetrahydrocannabinol (THC) and its metabolites in human urine sample, which’s used for detection and auxiliary diagnosis of drug addiction. This kit only provides tetrahydrocannabinol (THC) test results, and results obtained shall be used in combination with other clinical information for analysis.
2. PRODUCT SPECIFICATION
| Model No. | THC |
| Methodology | Colloidal Gold |
| Sample Type | Urine |
| Time to Result | 3-8mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE |


| MAIN KIT COMPONENTS: *Test device *Disposable pipette *Instructions for Use |
Packing: *25 test /kit *Aluminum foil bag labeling *shrink wrap |
3.TEST METHOD
| 1 |
Remove the reagent card from the foil bag and lay it flat on a level work surface and label it
|
| 2 |
Use disposable pipette to pipette urine sample, discard first two drops of urine sample,add 3 drops (approx. 100μL) of bubble-free urine sample dropwise to well of test device vertically and slowly, and start counting time
|
| 3 |
The results should be interpreted within 3-8 minutes, after 8 minutes the test results are invalid.
|
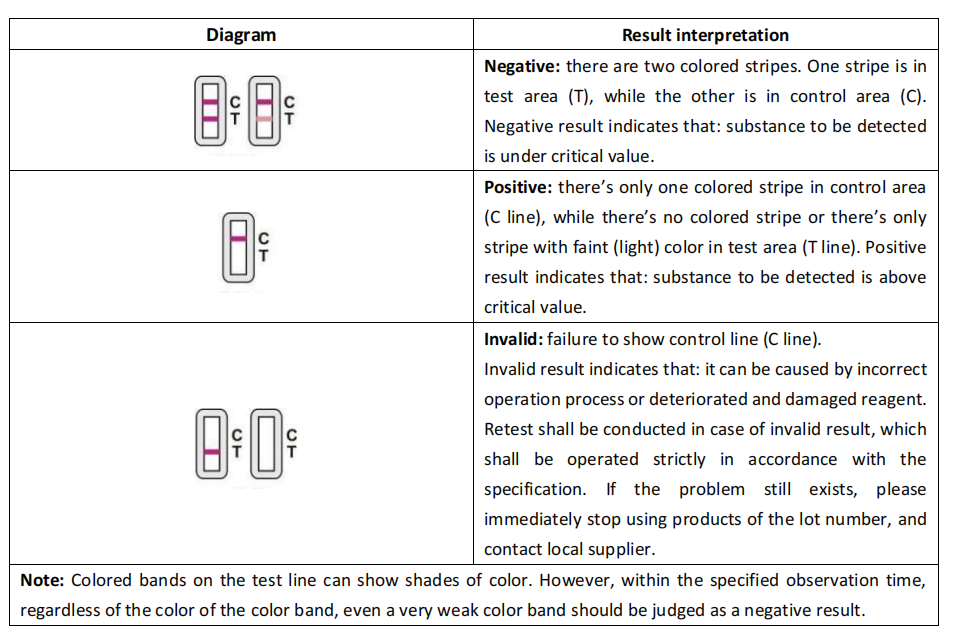
4.Result reading
5. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration