Rotavirus And Adenovirus Antigen Combo Stool Latex Test kit

1. INTEDED USE
This kit is applicable to qualitative detection of species A rotavirus or adenovirus antigen that may exist in human stool sample, which’s suitable for auxiliary diagnosis of species A rotavirus and adenovirus infection of infantile diarrhea patients. This kit only provides species A rotavirus and adenovirus antigen test results, and results obtained shall be used in combination with other clinical information for analysis.
2. PRODUCT SPECIFICATION
| Model No. | RV/AV |
| Methodology | Colloidal Gold |
| Sample Type | Faces |
| Time to Result | 10-15mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485, CE Certificate, UCKA MHRA Certificate |

|
MAIN KIT COMPONENTS * Test device * Sample collection tube * Disposable pipette * Instructions for Use |
PACKING * 25 test /kit * Aluminum foil bag labeling * shrink wrap
|
3.TEST METHOD

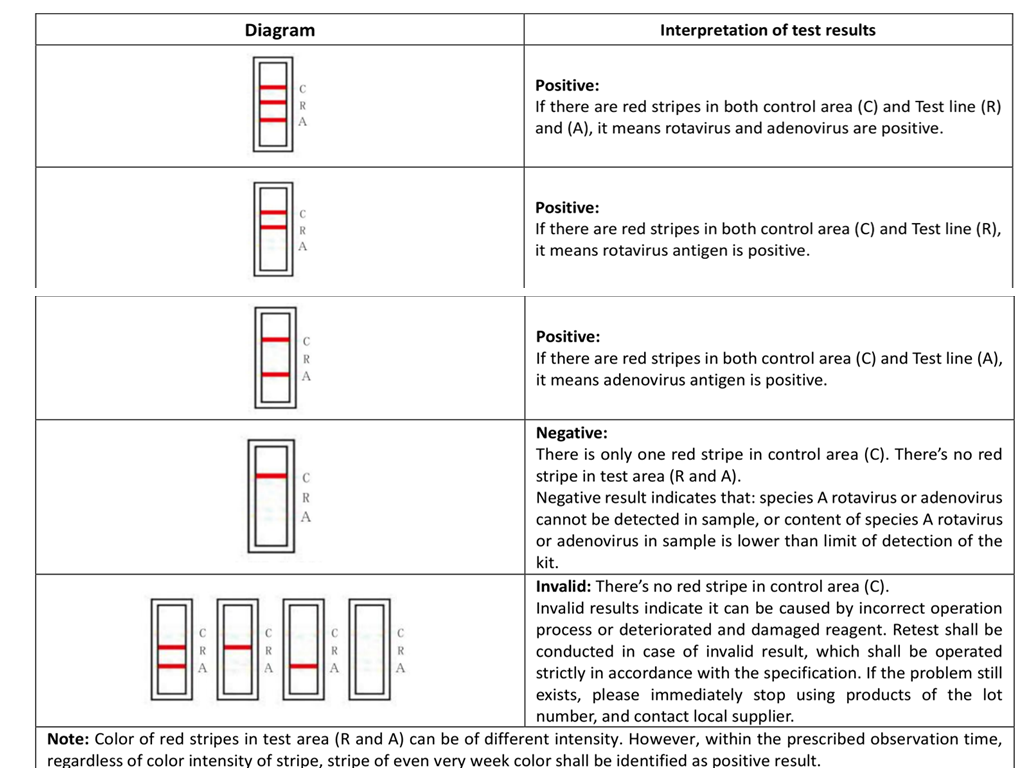
4.RESULT EVALUATION AND EXPLANATION
5.CLINICAL PERFORMANCE
Clinical performance of this product’s assessed through collection of 293 cases of clinical samples. Sample providers include patients infected with species A rotavirus and adenovirus and normal subjects. Marketed kit of colloidal gold methods used as reference reagent. In comparison of BAYSEN reagent detection with reference reagent:
| Baysen Result of RV/AV |
Test result of Reference reagent | Positive coincidence rate: 99.36% (95%C.I. 96.48%~99.89%) Negative coincidence rate: 100.00% (95%C.I. 97.25%~100.00%) Total coincidence rate: 99.66% (95%C.I. 98.09%~99.94%) |
||
| Positive | Negative | Total | ||
| Positive | 156 | 0 | 156 | |
| Negative | 1 | 136 | 137 | |
| Total | 157 | 136 | 293 | |
6. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration