Quantitative Estradiol Diagnostic Kit
1. INTEDED USE
This kit is applicable to in vitro quantitative detection of estradiol (E2) in human serum/plasma/whole blood sample, which’s mainly used for assessment of ovarian function. This kit only provides estradiol (E2) test results, and results obtained shall be used in combination with other clinical information for analysis. It must only be used by healthcare professionals.
2. PRODUCT SPECIFICATION
| Model No. | E2 |
| Methodology | Fluorescence Immunochromatographic Assay |
| Sample Type | serum/plasma/whole blood |
| Time to Result | 10-15mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE,MHRA |


| MAIN KIT COMPONENTS: *Test device *solution *Instructions for Use |
Packing: *25 test /kit *Aluminum foil bag labeling *shrink wrap |
3.TEST METHOD
| 1 |
Open the aluminum foil bag package of reagent, and take out the test device;
|
| 2 |
Horizontally insert the test device into the slot of immune analyzer;
|
| 3 |
On home page of operation interface of immune analyzer, click “Standard” to enter test interface;
|
| 4 |
Click “QC Scan” to scan the QR code on inner side of the kit; input kit related parameters into instrument, and select sample type;
Note: Each batch number of the kit shall be scanned for one time. If the batch number has been scanned, then skip this step. |
| 5 |
Check the consistency of “Product Name”, “Batch Number” etc. on test interface with information on the kit label;
|
| 6 | Take out sample diluent upon consistent information, add 80μL serum/plasma/whole blood sample, and thoroughly mix them; |
| 7 | Add 80µL aforesaid thoroughly mixed solution into well of test device; |
| 8 | After complete sample addition, click “Timing” and remaining test time will be automatically displayed on the interface. |
| 9 |
Immune analyzer will automatically complete test and analysis when test time is reached.
|
| 10 |
Ⅰ-2: Result calculation and display
After test by immune analyzer is completed, test result will be displayed on test interface or can be viewed through “History” on home page of operation interface. |
4.Product Performance
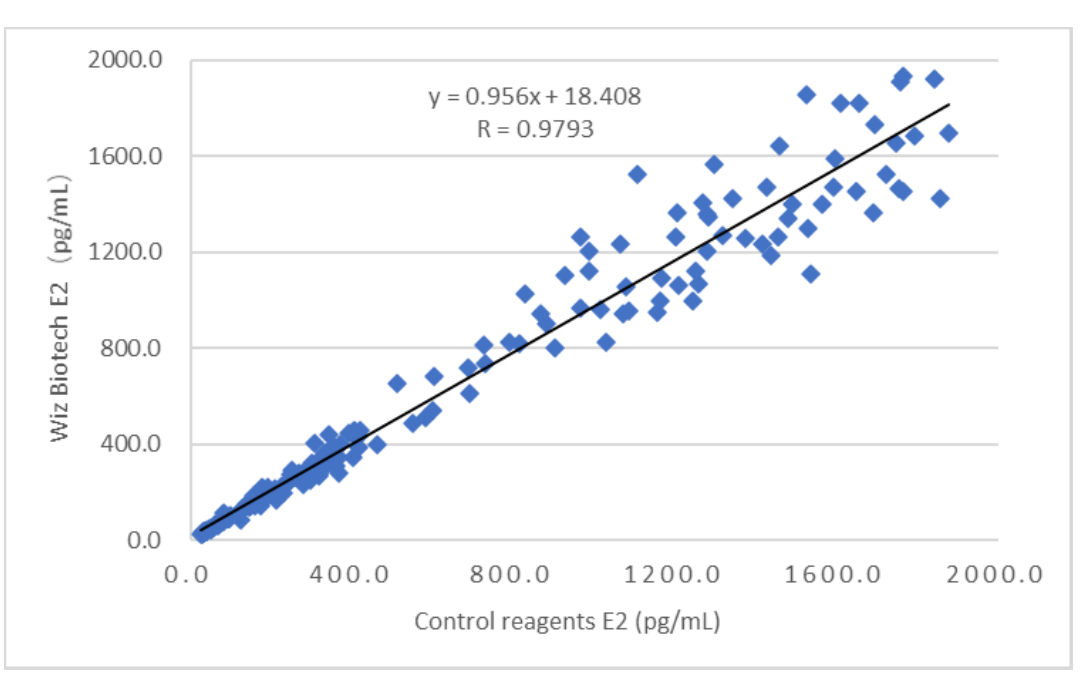
Clinical performance of this product’s assessed through collection of 170 cases of clinical samples. Marketed kit of electrochemiluminescence’s used as reference reagent. Detection results have been compared and their comparability has been studied through linear regression, and correlation coefficients of the two assays are Y=0.956X+18.408 and R=0.9793 respectively.
5. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration