Malaria PF PV rapid test uncut sheet
1. INTEDED USE
This kit is applicable to in vitro qualitative detection of antigen to plasmodium falciparum histidine-rich proteins II (HRPII) and antigen to plasmodium vivax lactate dehydrogenase (pvLDH) in human whole blood sample, and it’s used for auxiliary diagnosis of plasmodium falciparum (pf) and plasmodium vivax (pv) infection. This kit only provides detection result of antigen to plasmodium falciparum histidine-rich proteinsII and antigen to plasmodium vivax lactate dehydrogenase.
2. PRODUCT SPECIFICATION
| Model No. | Malaria PF PV Uncut sheet |
| Methodology | Colloidal Gold |
| Sample Type | whole blood |
| Time to Result | 10-15mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485, CE Certificate, UCKA MHRA Certificate |

|
Feature • High sensitive • result reading in 15-20 minutes • Easy operation • High Accuracy |
PACKING * 20 bags /CTN * Aluminum foil bag labeling * shrink wrap
|
3.TEST METHOD
| 1 |
.Restore sample and kit to room temperature, take test device out of the sealed pouch, and lie it on horizontal bench.
|
| 2 | Pipette 1 drop (around 5μL) of whole blood sample into the well of test device (‘S’ well) vertically and slowly by the disposable pipette provided. |
| 3 |
Turn sample diluent upside down, discard first two drops of sample diluent, add 3-4 drops of bubble-free sample diluent dropwise to the well of test device (‘D’ well)vertically and slowly, and start counting time. |
| 4 |
Result shall be interpreted within 15~ 20 minutes, and detection result is invalid after 20 minutes.
|
4.RESULT EVALUATION AND EXPLANATION
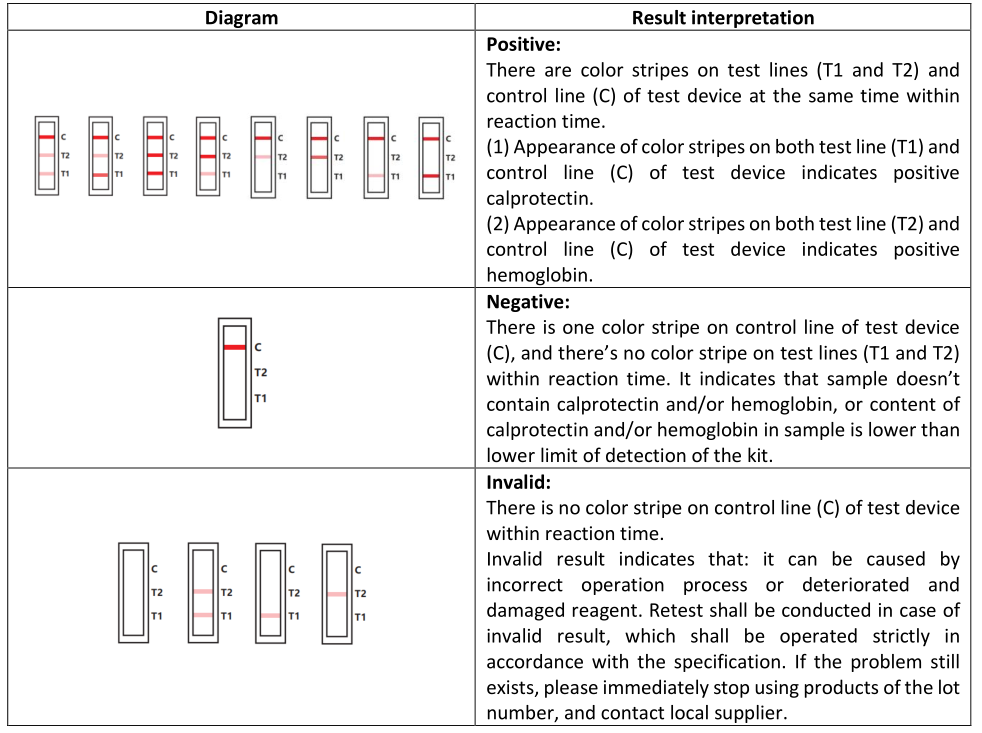
5. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration