Qantitative Free Thyroxine Diagnostic Kit
1. INTEDED USE
This kit is applicable to in vitro quantitative detection of free thyroxine (FT4) in human serum/plasma/whole blood sample, which’s mainly used for assessment of thyroid function. This kit only provides free thyroxine (FT4) test results, and results obtained shall be used in combination with other clinical information for analysis.
2. PRODUCT SPECIFICATION
| Model No. | FT4 |
| Methodology | Fluorescence Immunochromatographic Assay |
| Sample Type | Serum/Plasma/Whole Blood |
| Time to Result | 10-15mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE,MHRA |

| MAIN KIT COMPONENTS
* Test device * Sample diluents * Instructions for Use |
PACKING
* 25 test /kit * Aluminum foil bag labeling * shrink wrap |
3.TEST METHOD
4.CLINICAL PERFORMANCE
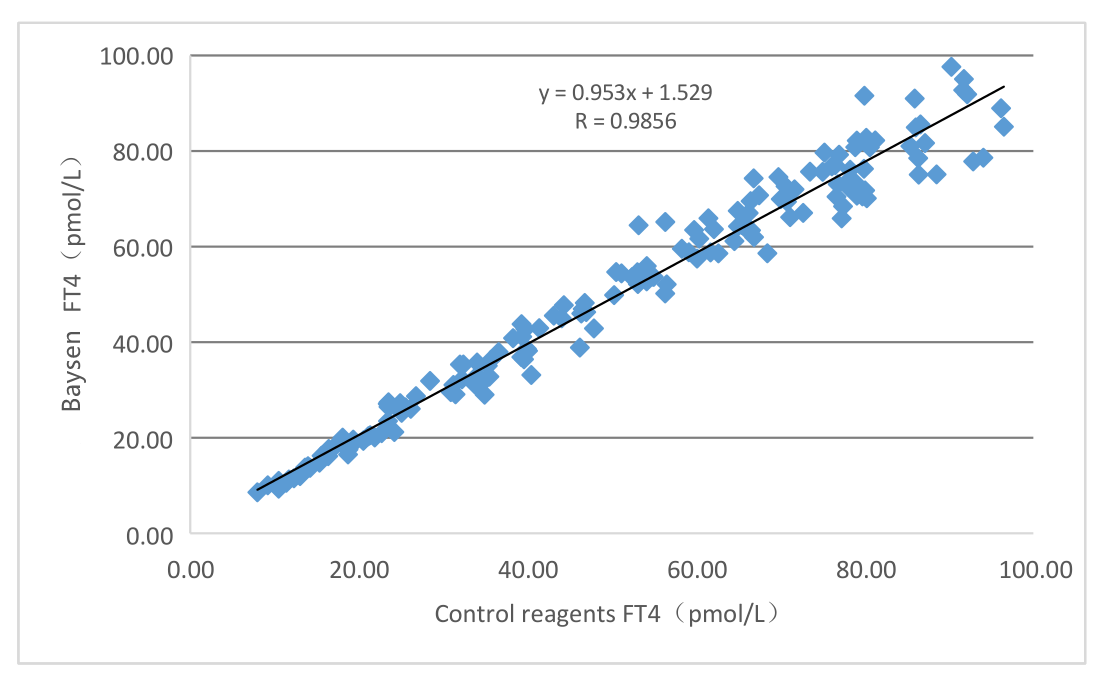
Clinical performance of this product’s assessed through collection of 161 cases of clinical samples. Corresponding marketed kit of electrochemiluminescence assays used as reference reagent, detection results have been compared and their comparability has been studied through linear regression, and correlation coefficients of the two assays are Y=0.953X+1.529 and R=0.9856 respectively.
5. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration