Diagnostic Kit for malaria P.F/P.V rapid test
1. INTEDED USE
This kit is applicable to in vitro qualitative detection of antigen to plasmodium falciparum histidine-rich proteins II(HRPII) and antigen to plasmodium vivax lactate dehydrogenase (pvLDH) in human whole blood sample, and it’s used for auxiliary diagnosis of plasmodium falciparum (pf) and plasmodium vivax (pv) infection. This kit only provides detection result of antigen to plasmodium falciparum histidine-rich proteinsII and antigen to plasmodium vivax lactate dehydrogenase, and results obtained shall be used in combination with other clinical information for analysis.
2. PRODUCT SPECIFICATION
| Model No. | Malaria P.F/P.V |
| Methodology | Colloidal Gold |
| Sample Type | whole blood |
| Time to Result | 10-15 minutes. |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE |
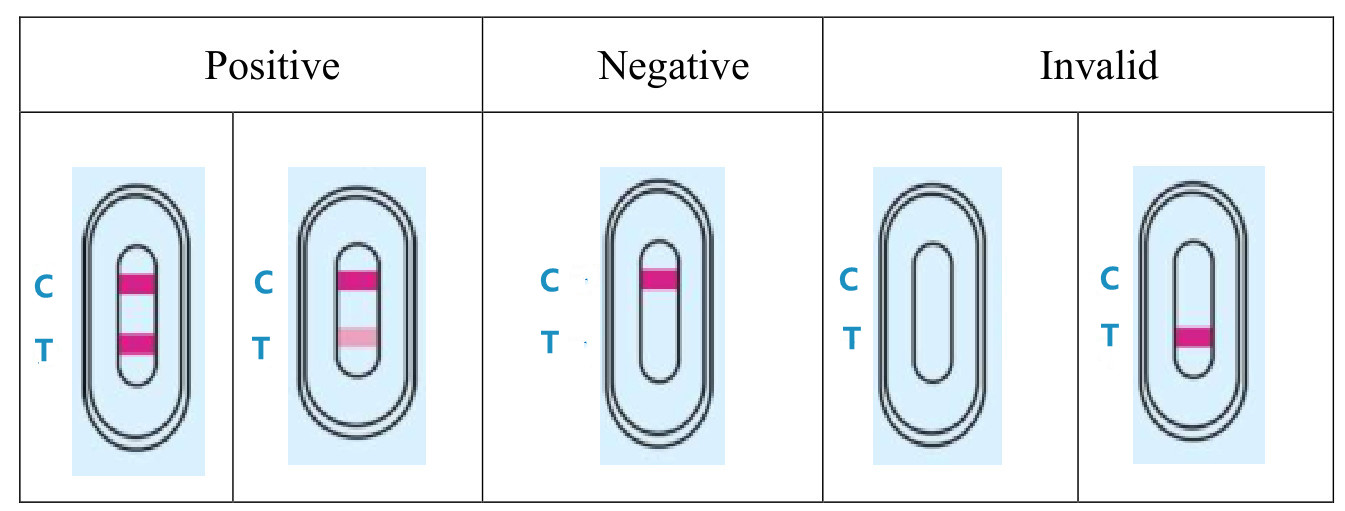
3.RESULT EVALUATION AND EXPLANATION
4.FACTORY PROFILE

We take part in exhibition all over the world to set cooperation with client.

5. GLOBAL PARTNER