Diagnostic Kit for IgM Antibody to Mycoplasma Pneumoniae
1. INTEDED USE
This kit is applicable to in vitro qualitative detection of antibody to chlamydia pneumoniae in human serum/plasma/whole blood sample, and it’s used for auxiliary diagnosis of chlamydia pneumoniae infection. This kit only provides test results of IgM antibody to chlamydia pneumoniae.
2. PRODUCT SPECIFICATION
| Model No. | MP-IGM |
| Methodology | Colloidal Gold |
| Sample Type | whole blood |
| Time to Result | 10-15 minutes. |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE |
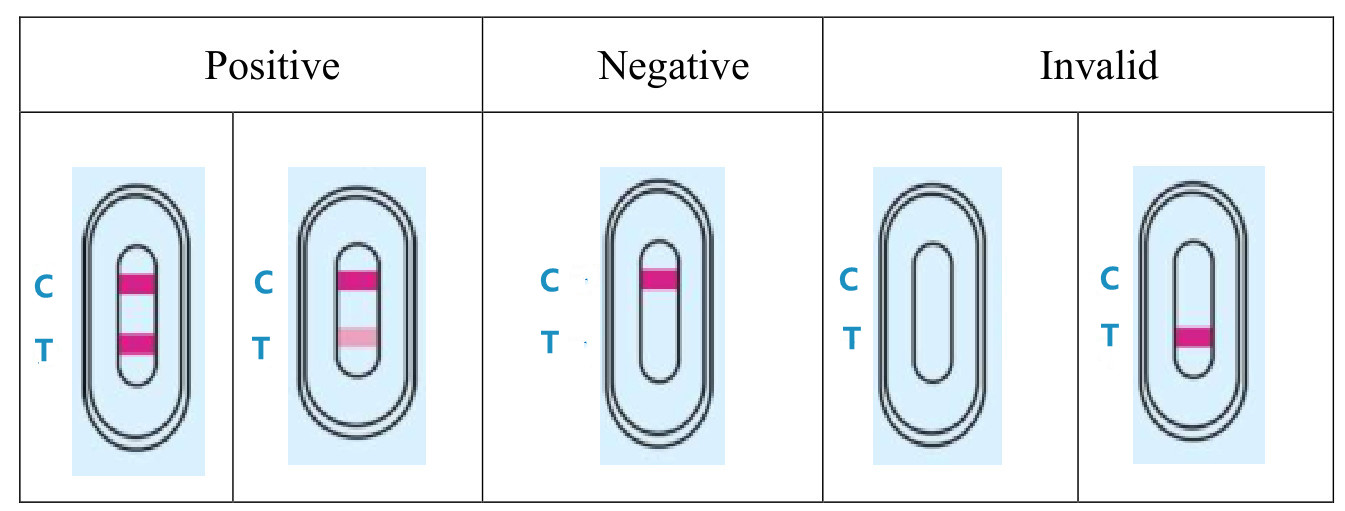
3.RESULT EVALUATION AND EXPLANATION
4.FACTORY PROFILE

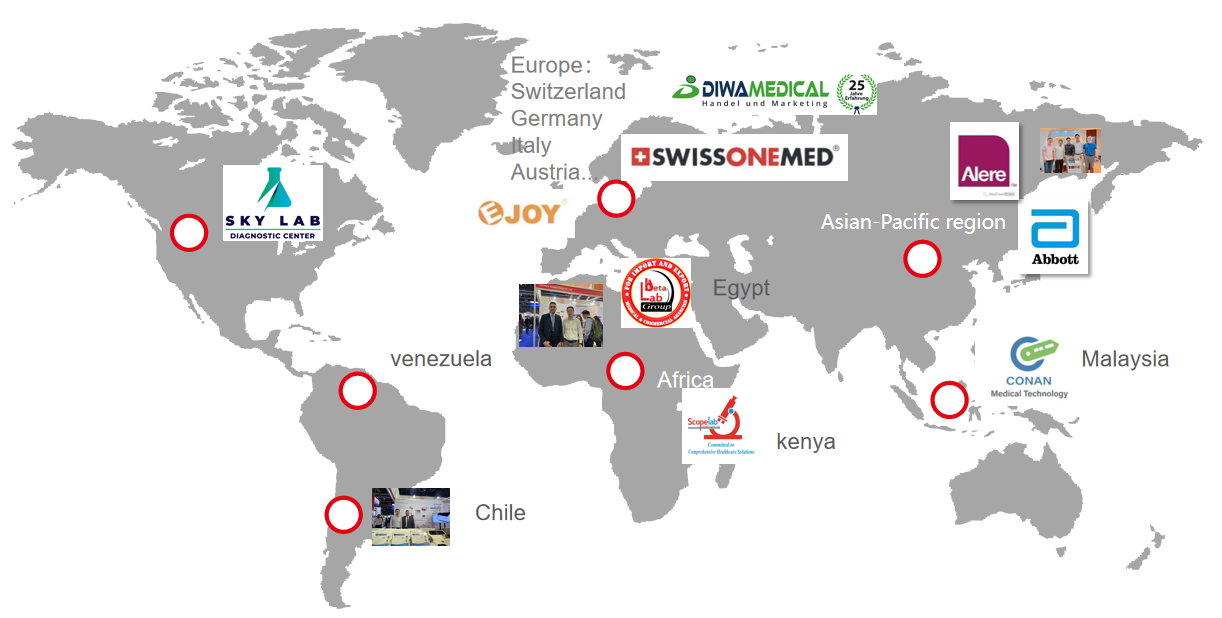
We take part in exhibition all over the world to set cooperation with client.

5. GLOBAL PARTNER
Write your message here and send it to us