Diagnostic Kit for IgM Antibody to C. Pneumoniae
1. INTEDED USE
This kit is applicable to in vitro qualitative detection of antibody to chlamydia pneumoniae in human serum/plasma/whole blood sample, and it’s used for auxiliary diagnosis of chlamydia pneumoniae infection. This kit only provides test results of IgM antibody to chlamydia pneumoniae.
2. PRODUCT SPECIFICATION
| Model No. | CPN-IGM |
| Methodology | Colloidal Gold |
| Sample Type | whole blood |
| Time to Result | 10-15 minutes. |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE |
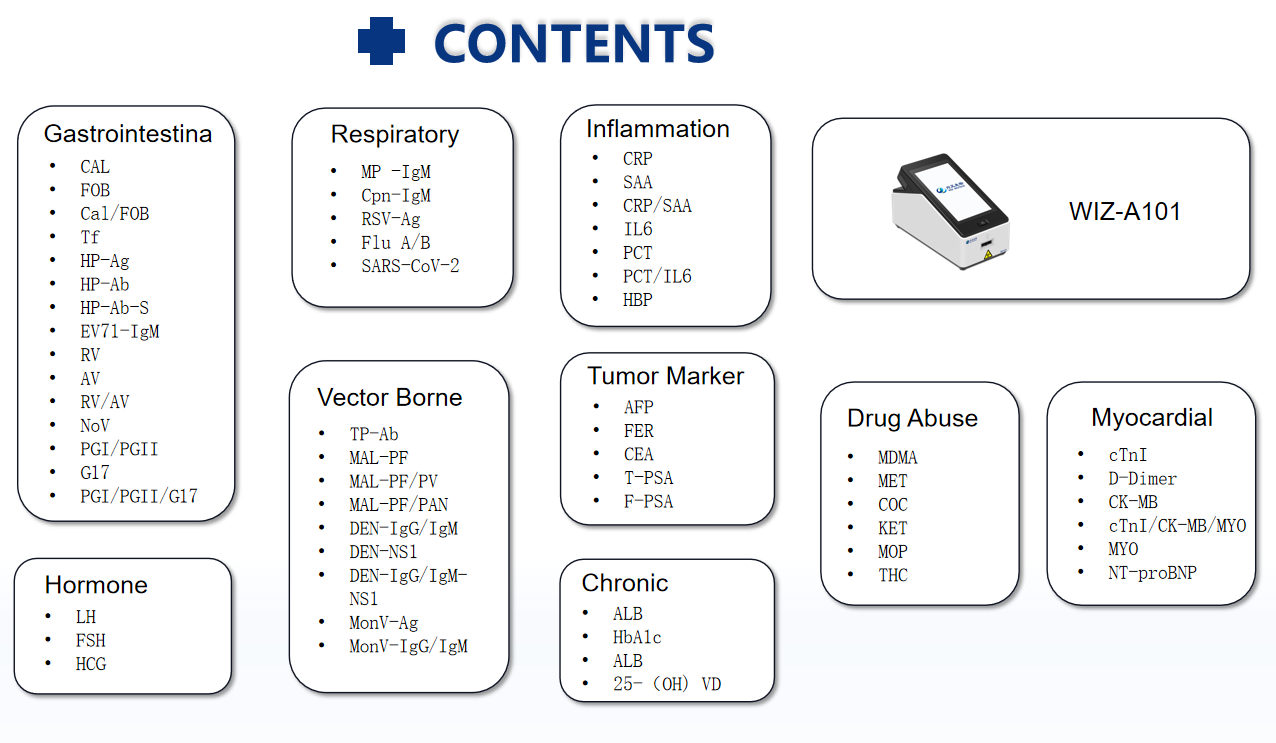
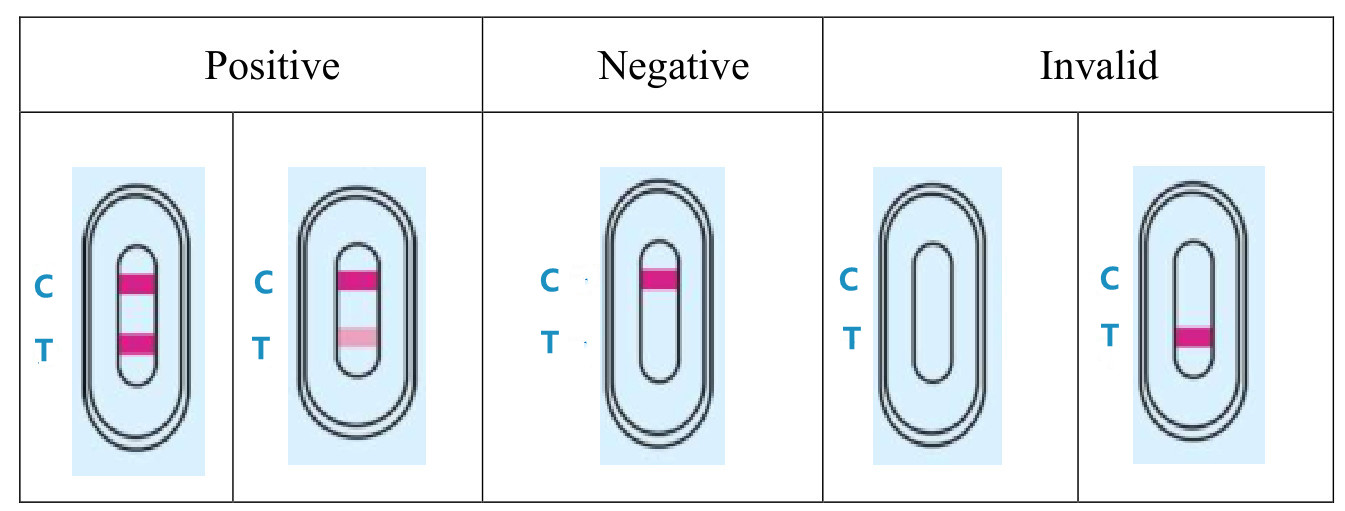
3.RESULT EVALUATION AND EXPLANATION
4.FACTORY PROFILE

We take part in exhibition all over the world to set cooperation with client.

5. GLOBAL PARTNER