Diagnostic Kit for Antigen to Helicobacter Pylori(Latex)
1. INTEDED USE
This kit is intended for in vitro qualitative detection of antigen to helicobacter pylori in human stool sample, which’s suitable for auxiliary diagnosis of helicobacter pylori infection. This kit only provides detection result of antigen to helicobacter pylori, and results obtained shall be used in combination with other clinical information for analysis. It must only be used by healthcare professionals.
2. PRODUCT SPECIFICATION
| Model No. | HP-AG |
| Methodology | Latex |
| Sample Type | Faces |
| Time to Result | 10-15 minutes. |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE Certificate,UCKA MHRA |

| MAIN KIT COMPONENTS* Test device
* Disposable pipette * Sample collection tubes * Instructions for Use |
PACKING
* 25 test /kit * Aluminum foil bag labeling * Shrink wrap |
3.TEST METHOD
| 1 | Use sample collection tubes for sample collection, thorough mixing and dilution for later use. Use proof stick to take 30mg of stool, place it in Sample collection tubes loaded with sample diluent, screw the cap tightly, and thoroughly shake it for later use. |
| 2 | In case of thin stool of patients with diarrhea, use disposable pipette to pipette sample, and add 3 drops (approx.100μL) of sample dropwise to Sample collection tubes, and thoroughly shake sample and sample diluent for later use. |
| 3 | Remove test device from aluminum foil pouch, lie it on a horizontal workbench, and do a good job in marking. |
| 4 | Discard first two drops of diluted sample, add 3 drops (approx. 100μL) of bubble-free diluted sample dropwise to well of test device vertically and slowly, and start counting time. |
| 5 | Interpret result within 10-15 minutes, and detection result is invalid after 15 minutes (see detailed results in result interpretation). |
4.RESULT EVALUATION AND EXPLANATION
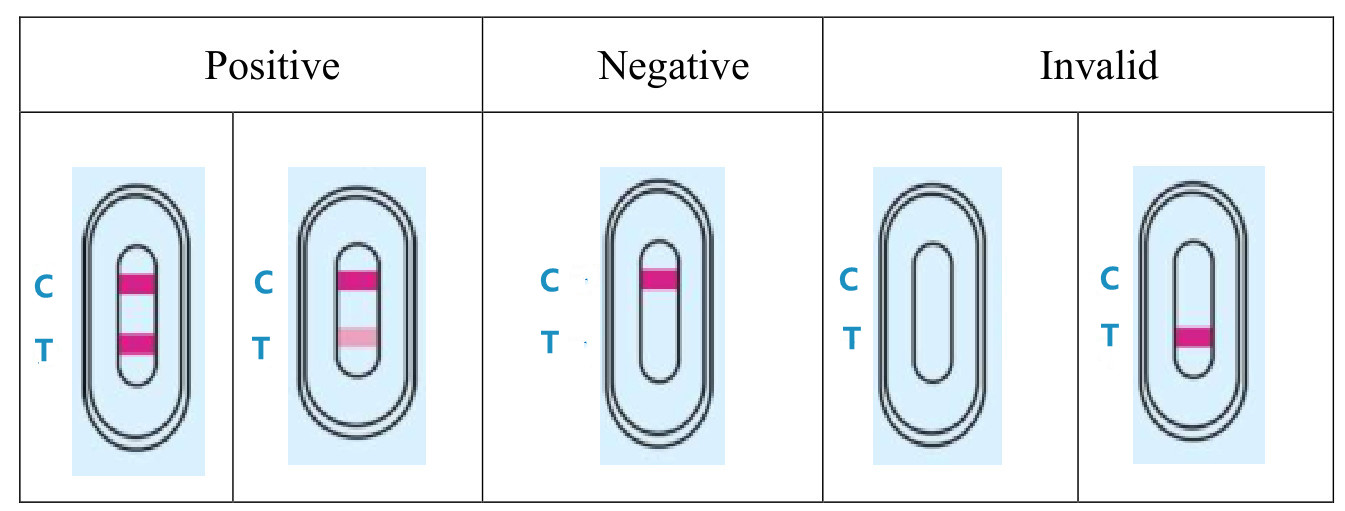
5.CLINICAL PERFORMANCE
Clinical performance of this product’s assessed through collection of 315 cases of clinical samples. Sample providers include patients infected with helicobacter pylori and normal subjects. Use marketed kit of turbidimetric inhibition immunoassays as reference reagent to compare with Baysen Reagent
| Baysen Result of HP-AG | Test result of Reference reagent | Positive coincidence rate:98.91% (95%C.I. 96.12%~99.70%) Negative coincidence rate 100.00% (95%C.I. 97.15%~100.00%) Total coincidence rate:99.37% (95%C.I. 97.71%~99.83%) |
||
| Positive | Negative | Total | ||
| Positive | 182 | 0 | 182 | |
| Negative | 2 | 131 | 133 | |
| Total | 184 | 131 | 315 | |
6. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration