Diagnostic Kit for Antibody to Helicobacter Pylori
1. INTEDED USE
This kit is applicable to in vitro qualitative detection of antibody to H.pylori (HP) in human whole blood, serum or plasma sample, which’s suitable for auxiliary diagnosis of HP infection. This kit only provides test results of antibody to H.pylori (HP), and results obtained shall be used in combination with other clinical information for analysis.
2. PRODUCT SPECIFICATION
| Model No. | HP-AB |
| Methodology | Colloidal Gold |
| Sample Type | Serum/Plasma/Whole Blood |
| Time to Result | 10-15mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485, CE Certificate, UCKA MHRA Certificate |

|
MAIN KIT COMPONENTS * Test device * Sample collection tube * Disposable pipette * Instructions for Use |
PACKING * 25 test /kit * Aluminum foil bag labeling * shrink wrap
|
3.TEST METHOD
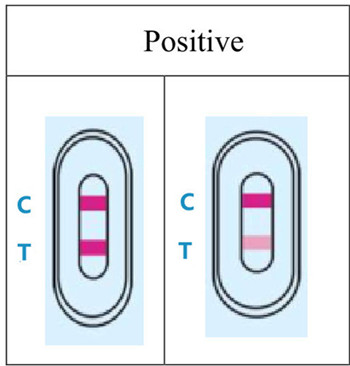
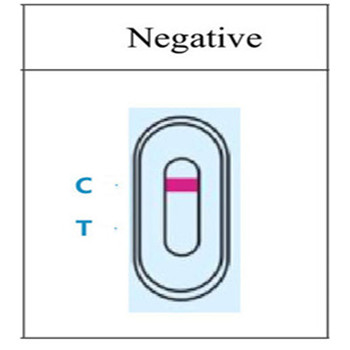
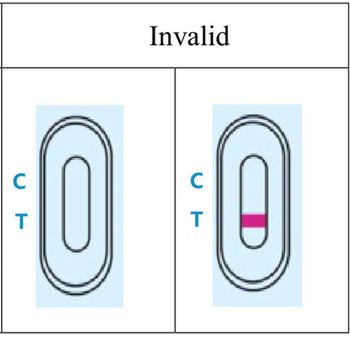
4.RESULT EVALUATION AND EXPLANATION
5.CLINICAL PERFORMANCE
Clinical performance of this product’s assessed through collection of 331 cases of clinical samples. Sample providers include patients infected with helicobacter pylori and normal subjects. Marketed kit of colloidal gold methods used as reference reagent, in comparison of Baysen reagent detection with reference reagent:
| Baysen Result of RV/AV |
Test result of Reference reagent | Positive coincidence rate: 98.92% (95%C.I. 96.16%~99.70%) Negative coincidence rate: 100.00% (95%C.I. 97.42%~100.00%) Total coincidence rate: 99.40% (95%C.I. 97.82%~99.83%) |
||
| Positive | Negative | Total | ||
| Positive | 184 | 0 | 184 | |
| Negative | 2 | 145 | 147 | |
| Total | 186 | 145 | 331 | |
6. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration