Diagnostic kir for Alpha-fetoprotein rapid test
1. INTEDED USE
This kit is applicable to the in vitro quantitative detection of alpha-fetoprotein (AFP) in human serum/plasma/whole blood samples and used for auxiliary early diagnosis of primary hepatic carcinoma. The kit only provides test result of alpha-fetoprotein (AFP). The obtained result shall be analyzed in combination with other clinical information. I
2. PRODUCT SPECIFICATION
| Model No. | AFP |
| Methodology | Fluorescence Immunochromatographic Assay |
| Sample Type | serum/Plasma/Whole Blood |
| Time to Result | 10-15mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE,MHRA |

| MAIN KIT COMPONENTS
* Test device * Sample diluents * Instructions for Use |
PACKING
* 25 test /kit * Aluminum foil bag labeling * shrink wrap |
3.TEST METHOD
4.CLINICAL PERFORMANCE
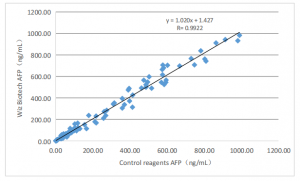
Clinical evaluation performance of the product is assessed through collecting 176 clinical samples. Use a corresponding marketed fluorescence immunochromatographic kit as the control reagent and compare the test results. Use linear regress to investigate their comparability.
The correlation coefficients of the two tests are Y=1.020X+1.427 and R=0.9922, respectively.
5. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration
6.EXHIBITION

7.GLOBAL PARTNER