Diagnostic kit for Prostate Specific Antigen
1. INTEDED USE
Diagnostic Kit for Prostate Specific Antigen (fluorescence immunochromatographic assay) is a fluorescence immunochromatographic assay for the quantitative detection of Prostate Specific Antigen (PSA) in human serum or plasma, which is mainly used to auxiliary diagnosis of prostatic disease.All positive sample must be confirmed by other methodologies.
2. PRODUCT SPECIFICATION
| Model No. | PSA |
| Methodology | Fluorescence Immunochromatographic Assay |
| Sample Type | serum/Plasma |
| Time to Result | 10-15mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE,MHRA |

| MAIN KIT COMPONENTS
* Test device * Sample diluents * Instructions for Use |
PACKING
* 25 test /kit * Aluminum foil bag labeling * shrink wrap |
3.TEST METHOD
4.OPERATION
1.Lay aside all reagents and samples to room temperature.
2.Open the Portable Immune Analyzer(WIZ-A101), enter the account password login according to the operation method of the instrument, and enter the detection interface.
Scan the dentification code to confirm the test item.
3.Take out the test card from the foil bag.
4.Insert the test card into the card slot, scan the QR code, and determine the test item.
5.Add80μL serum or plasma sample into sample diluent, and mix well.
6.Add 80μL sample solution to sample well of the card.
7.Click the "standard test" button, after 15 minutes, the instrumentwill automatically detect the test card, it can read the results from the display screen of the instrument, and record/print the test results.
8.Refer to the instruction of Portable Immune Analyzer(WIZ-A101).
5. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration
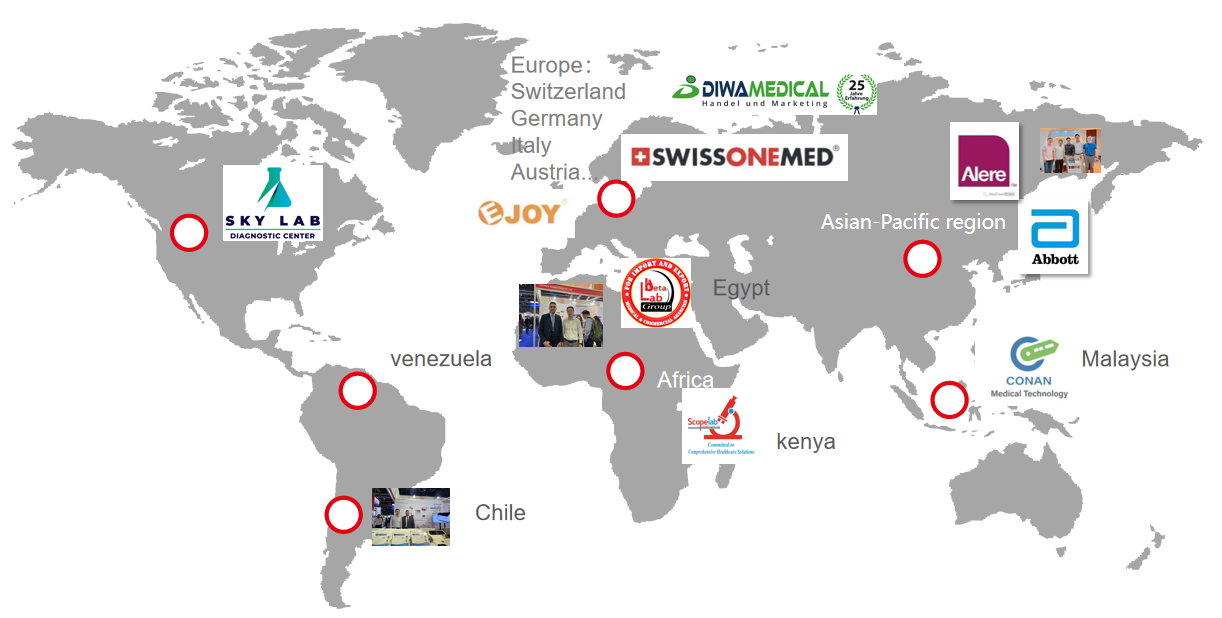
6.EXHIBITION

7.GLOBAL PARTNER