DiaBench ™ Rapid Blood Type & Infectious Combo Test
1. INTEDED USE
Blood Type (ABD) Rapid Test (Solid Phase) and Infectious Combo Test (Colloidal Gold) is suitable for ABD blood grouping system A/B antigen and Rh blood grouping system D antigen detection in human venous whole blood/fresh finger terminal blood and auxiliary diagnosis of hepatitis B virus, syphilis spirochete, human immunodeficiency virus, and hepatitis C virus infections, and is not suitable for blood screening. The results obtained should be analyzed in conjunction with other clinical information. It is intended for use by medical professionals only.
2. PRODUCT SPECIFICATION
| Model No. | ABD/HCV/HBSAG/HIV/TP-AB Combo |
| Methodology | Solid Phase/ Colloidal Gold |
| Sample Type | venous whole blood/fresh finger terminal blood |
| Time to Result | 10-15mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485 |

|
MAIN KIT COMPONENTS *Test device *Sample diluents *Disposable pipette *Instructions for Use |
Packing *20 test /kit *Aluminum foil bag labeling *shrink wrap
|
3.TEST METHOD
| 1 | Read the instruction for use and in strict conformity with instruction for use required operation to avoidaffecting the accuracy of the test results. |
| 2 | Before the test, the kit and the sample are taken out from the storage condition and balanced to room temperature and mark it. |
| 3 | Tearing the packaging of the aluminum foil pouch, take out the test device and mark it, then place it horizontally on the test table. |
| 4 | The sample to be tested (whole blood) was added to S1 and S2 wells with 2 drops (about 20ul), and to wells A,B and D with 1 drop (about 10ul), respectively. After the sample is added, 10-14 drops of sample dilution (about 500ul) are added to the Diluent wells and the timing is started. |
| 5 | Test results should be interpreted within 10~15 minutes, if more than 15min interpreted results are invalid. |
| 6 | Visual interpretation can be used in result interpretation. |
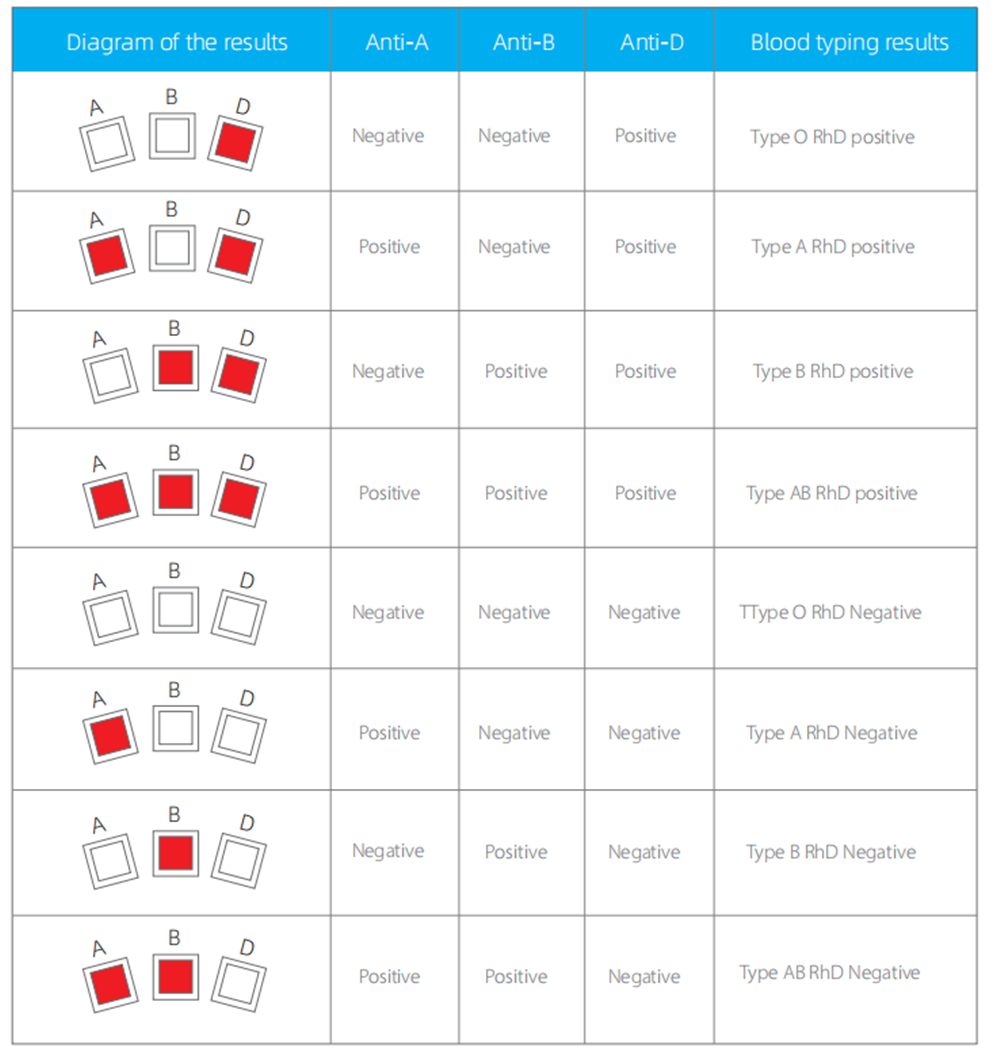
4.RESULT EVALUATION AND EXPLANATION
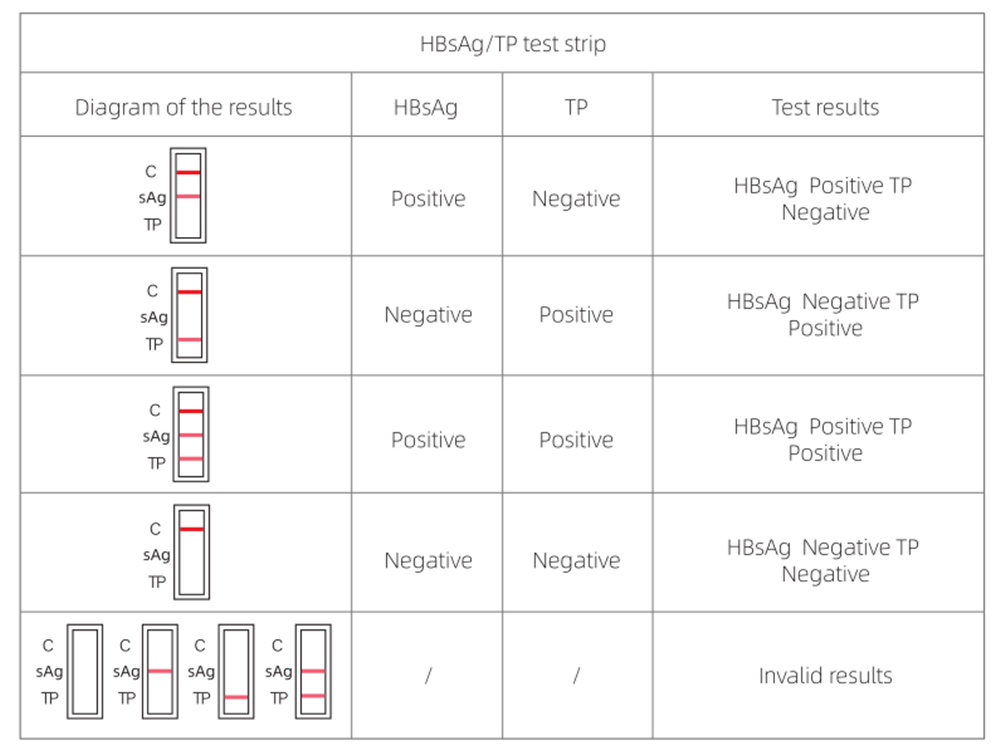
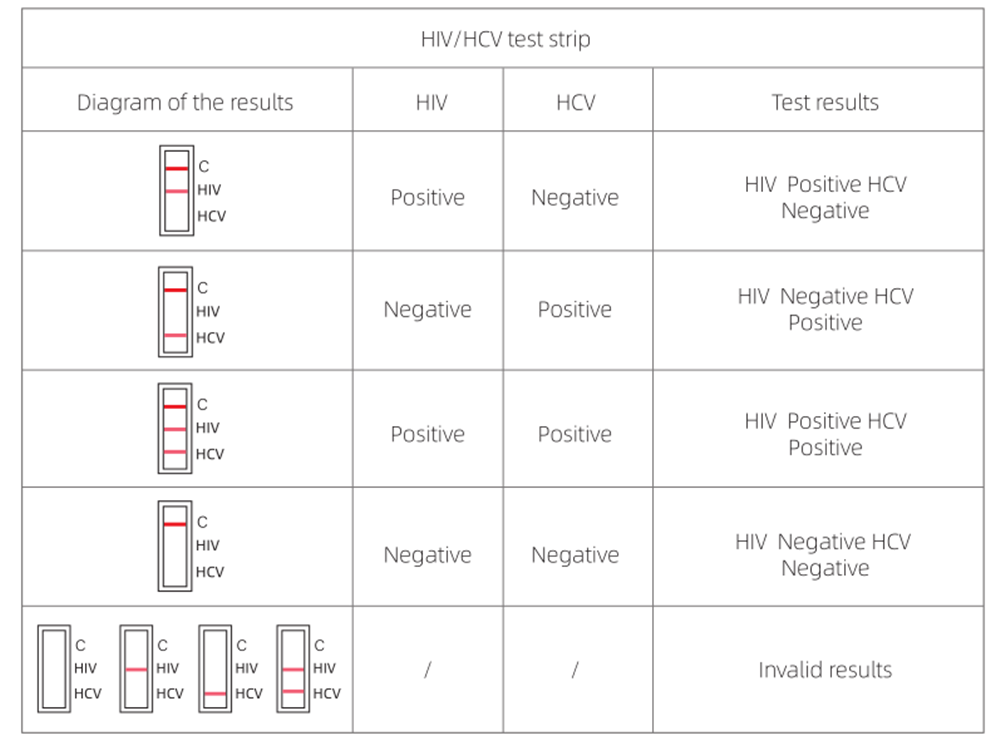
5.CLINICAL PERFORMANCE
| Baysen Result of HBsAg | Test result of Reference reagent | Positive coincidence rate:99.10% (95%C.I. 96.79%~99.75%) Negative coincidence rate:98.37% (95%C.I.96.24%~99.30%) Total coincidence rate:98.68% (95%C.I.97.30%~99.36%) |
||
| Positive | Negative | Total | ||
| Positive | 221 | 5 | 226 | |
| Negative | 2 | 302 | 304 | |
| Total | 223 | 307 | 530 | |
| Baysen Result of TP |
Test result of Reference reagent | Positive coincidence rate:95.71% (95%C.I. 90.97%~98.02%) Negative coincidence rate:97.69% (95%C.I.95.67%~98.78%) Total coincidence rate:97.17% (95%C.I.95.38%~98.28%) |
||
| Positive | Negative | Total | ||
| Positive | 134 | 9 | 143 | |
| Negative | 6 | 381 | 387 | |
| Total | 140 | 390 | 530 | |
| Baysen Result of HIV | Test result of Reference reagent | Positive coincidence rate:97.03% (95%C.I. 91.63%~98.98%) Negative coincidence rate:99.77% (95%C.I.98.69%~99.96%) Total coincidence rate:99.25% (95%C.I.98.08%~99.71%) |
||
| Positive | Negative | Total | ||
| Positive | 98 | 1 | 99 | |
| Negative | 3 | 428 | 431 | |
| Total | 101 | 429 | 530 | |
| Baysen Result of HCV | Test result of Reference reagent | Positive coincidence rate:93.84% (95%C.I. 85.22%~97.58%) Negative coincidence rate:99.35% (95%C.I.98.12%~99.78%) Total coincidence rate:98.68% (95%C.I.97.30%~99.36%) |
||
| Positive | Negative | Total | ||
| Positive | 61 | 3 | 64 | |
| Negative | 4 | 462 | 466 | |
| Total | 65 | 465 | 530 | |