Immunoassay Blood Gastrin 17 Test kit for Stomach Disease
1. INTEDED USE
This kit is intended for the in vitro quantitative detection the content of Gastrin 17 (G-17) in human serum/plasma/whole blood samples. This kit only provides the test result of Gastrin 17 (G-17). The obtained result shall be analyzed in combination with other clinical information.
2. PRODUCT SPECIFICATION
| Model No. | G-17 |
| Methodology | Fluorescence Immunochromatographic Assay |
| Sample Type | Serum/Plasma/Whole blood |
| Time to Result | 10-15mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE,MHRA |

| MAIN KIT COMPONENTS* Test device
* Sample diluents * Instructions for Use |
PACKING* 25 test /kit
* Aluminum foil bag labeling * shrink wrap |
3.TEST METHOD
4.CLINICAL PERFORMANCE
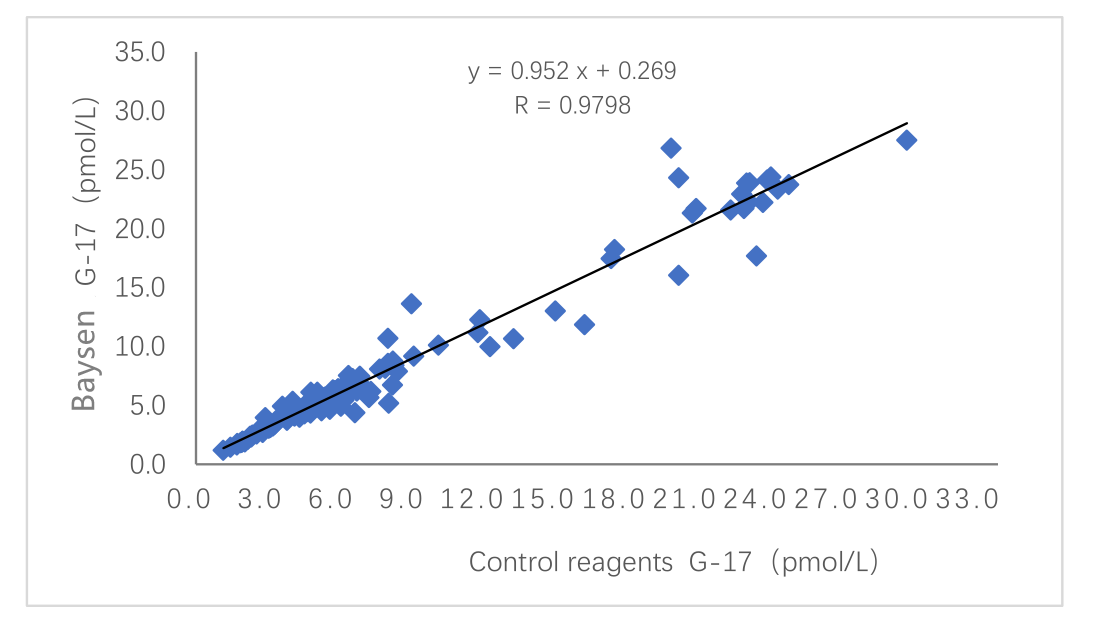
Clinical evaluation performance of the product is assessed through collecting 155 clinical samples. The results are compared by using the corresponding kit of marketed euzymelinked immunosorbent method as the reference reagent. Use linear regression to investigate their comparability. The correlation coefficients of the two tests are Y=0.952X+0.269 and R=0.9798, respectively.
5. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration








